Formula For Copper Ii Hydroxide
 | |
 | |
| Names | |
|---|---|
| IUPAC name Copper(II) hydroxide | |
| Other names Cupric hydroxide | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.039.817 |
| KEGG |
|
| PubChem CID |
|
| UNII |
|
| CompTox Dashboard (EPA) |
|
| InChI
| |
| SMILES
| |
| Properties | |
| Chemic formula | Cu(OH)2 |
| Molar mass | 97.561 g/mol |
| Appearance | Bluish or blue-greenish solid |
| Density | 3.368 g/cm3, solid |
| Melting point | 80 °C (176 °F; 353 K) estimate, decomposes into CuO |
| Solubility in water | negligible |
| Solubility product (Thousand sp) | 2.xx ten ten−20 [1] |
| Solubility | insoluble in ethanol; soluble in NH4OH |
| Magnetic susceptibility (χ) | +1170.0·10−half-dozen cmthree/mol |
| Thermochemistry | |
| Std tooth | 108 J·mol−1·K−1 |
| Std enthalpy of | −450 kJ·mol−i |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Skin, Eye, & Respiratory Irritant |
| NFPA 704 (fire diamond) | 2 0 0 |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LDl (median dose) | one thousand mg/kg (oral, rat) |
| NIOSH (US health exposure limits): | |
| PEL (Permissible) | TWA ane mg/mthree (as Cu)[2] |
| REL (Recommended) | TWA 1 mg/miii (as Cu)[ii] |
| IDLH (Firsthand danger) | TWA 100 mg/grand3 (as Cu)[2] |
| Safety data sheet (SDS) | SDS |
| Related compounds | |
| Other anions | Copper(II) oxide Copper(II) carbonate Copper(Ii) sulfate Copper(Two) chloride |
| Other cations | Nickel(Ii) hydroxide Zinc hydroxide Iron(II) hydroxide Cobalt hydroxide |
| Related compounds | Copper(I) oxide Copper(I) chloride |
| Except where otherwise noted, information are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Copper(Ii) hydroxide is the hydroxide of copper with the chemical formula of Cu(OH)2. It is a pale greenish blueish or blue green solid. Some forms of copper(II) hydroxide are sold equally "stabilized" copper(2) hydroxide, although they probable consist of a mixture of copper(2) carbonate and hydroxide. Cupric hydroxide is a potent base, although its low solubility in water makes this hard to find directly.
Occurrence [edit]
Copper(Two) hydroxide has been known since copper smelting began effectually 5000 BC although the alchemists were probably the first to industry it by mixing solutions of lye (sodium or potassium hydroxide) and blue vitriol (copper(II) sulfate).[3] Sources of both compounds were available in artifact.
It was produced on an industrial scale during the 17th and 18th centuries for use in pigments such as blue verditer and Bremen green.[iv] These pigments were used in ceramics and painting.[5]
Mineral [edit]
The mineral of the formula Cu(OH)2 is called spertiniite. Copper(II) hydroxide is rarely found as an uncombined mineral because it slowly reacts with carbon dioxide from the temper to form a basic copper(II) carbonate. Thus copper slowly acquires a deadening green blanket in moist air by the reaction:
- 2 Cu(OH)ii + COtwo → CutwoCOthree(OH)2 + H2O
The green cloth is in principle a ane:ane mole mixture of Cu(OH)2 and CuCO3.[6] This patina forms on bronze and other copper alloy statues such as the Statue of Liberty.
Product [edit]
Copper(II) hydroxide tin be produced by adding sodium hydroxide to a solution of a soluble copper(II) salt, such as copper(Two) sulfate (CuSO4·5HiiO):[7]
- 2NaOH + CuSO4·5H2O → Cu(OH)2 + 6H2O + Na2Then4
The precipitate produced in this manner, however, often contains water and an appreciable corporeality of sodium-containing impurities. Furthermore, this form of copper hydroxide tends to convert to black copper(II) oxide:[8]
- Cu(OH)2 → CuO + H2O
A purer product can be attained if ammonium chloride is added to the solution beforehand to generate ammonia in situ.[9] Alterrnatively information technology tin be produced in a two-pace procedure from copper(II) sulfate via "basic copper sulfate:"[eight]
- 4 CuSO4 + half dozen NH3 + 6H2O → Cu4SOiv(OH)half dozen + 3 (NH4)2And sofour
- Cu4SO4(OH)vi + two NaOH → iv Cu(OH)2 + NaiiSO4
Alternatively, copper hydroxide is readily made by electrolysis of water (containing a footling electrolyte such as sodium sulfate or magnesium sulfate) with a copper anode:
- Cu + 2OH− → Cu(OH)2 + 2e−
Structure [edit]
The construction of Cu(OH)two has been determined by X-ray crystallography The copper center is square pyramidal. Four Cu-O distances in the plane range are 1.96 Å, and the axial Cu-O distance is two.36 Å. The hydroxide ligands in the airplane are either doubly bridging or triply bridging.[10]
Reactions [edit]
It is stable to about 100 °C.[7]
Copper(2) hydroxide reacts with a solution of ammonia to form a deep blue solution of tetramminecopper [Cu(NHiii)four]two+ circuitous ion.
Copper(2) hydroxide catalyzes the oxidation of ammonia solutions in presence of dioxygen, giving ascension to copper ammine nitrites, such as Cu(NO2)2(NH3)n.[11] [12]
Copper(2) hydroxide is mildly amphoteric. It dissolves slightly in concentrated brine, forming [Cu(OH)4]2−.[xiii] [7]
Reagent for organic chemistry [edit]
Copper(II) hydroxide has a rather specialized function in organic synthesis. Oft, when it is utilized for this purpose, information technology is prepared in situ by mixing a soluble copper(Ii) salt and potassium hydroxide.
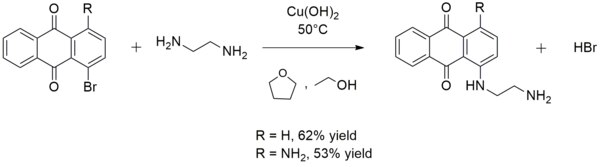
It is sometimes used in the synthesis of aryl amines. For instance, copper(2) hydroxide catalyzes the reaction of ethylenediamine with 1-bromoanthraquinone or 1-amino-4-bromoanthraquinone to class 1-((two-aminoethyl)amino)anthraquinone or ane-amino-4-((two-aminoethyl)amino)anthraquinone, respectively:[fourteen]
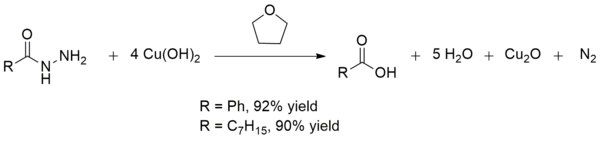
Copper(II) hydroxide besides converts acid hydrazides to carboxylic acids at room temperature. This conversion is useful in the synthesis of carboxylic acids in the presence of other fragile functional groups. The yields are by and large excellent as is the case with the production of benzoic acrid and octanoic acid:[xiv]
Uses [edit]
Copper(II) hydroxide in ammonia solution, known as Schweizer'south reagent, possesses the interesting ability to dissolve cellulose. This holding led to it existence used in the production of rayon, a cellulose fiber.
It is also used widely in the aquarium manufacture for its ability to destroy external parasites in fish, including flukes, marine ich, Brooklynellosis, and marine velvet, without killing the fish. Although other h2o-soluble copper compounds tin be effective in this function, they generally result in high fish mortality.
Copper(Ii) hydroxide has been used every bit an culling to the Bordeaux mixture, a fungicide and nematicide.[15] Such products include Kocide 3000, produced past Kocide L.Fifty.C. Copper(II) hydroxide is also occasionally used as ceramic colorant.
Copper(II) hydroxide has been combined with latex pigment, making a production designed to command root growth in potted plants. Secondary and lateral roots thrive and expand, resulting in a dense and good for you root arrangement. It was sold under the name Spin Out, which was outset introduced past Griffin Fifty.Fifty.C. The rights are now owned by SePRO Corp.[sixteen] It is now sold as Microkote either in a solution you utilise yourself, or as treated pots.
Other copper(Ii) hydroxides [edit]

Chemical construction of azurite, one of many copper(Ii) hydroxides (colour lawmaking: red = O, green = Cu, gray = C, white = H).[17]
Together with other components, copper(II) hydroxides are numerous. Several copper(Ii)-containing minerals contain hydroxide. Notable examples include azurite, malachite, antlerite, and brochantite. Azurite (2CuCO3·Cu(OH)2) and malachite (CuCO3·Cu(OH)2) are hydroxy-carbonates, whereas antlerite (CuSO4·2Cu(OH)ii) and brochantite (CuSOiv·3Cu(OH)ii) are hydroxy-sulfates.
Many synthetic copper(II) hydroxide derivatives have been investigated.[18]
References [edit]
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0150". National Establish for Occupational Safety and Health (NIOSH).
- ^ Richard Cowen, Essays on Geology, History, and People, Affiliate 3: "Fire and Metals: Copper".
- ^ Tony Johansen, Historic Artist's Pigments Archived 2009-06-09 at the Wayback Machine. PaintMaking.com. 2006.
- ^ Bluish verditer Archived 2007-09-27 at the Wayback Machine. Natural Pigments. 2007.
- ^ Masterson, Due west. L., & Hurley, C. N. (2004). Chemistry: Principles and Reactions, 5th Ed. Thomson Learning, Inc. (p 331)"
- ^ a b c O. Glemser and H. Sauer "Copper(Ii) Hydroxide" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited past 1000. Brauer, Academic Press, 1963, NY. Vol. two. p. 1013.
- ^ a b Solomon, Emerge D.; Rutkowsky, Susan A.; Mahon, Megan L.; Halpern, Erica Thousand. (2011). "Synthesis of Copper Pigments, Malachite and Verdigris: Making Tempera Paint". Journal of Chemical Education. 88 (12): 1694–1697. Bibcode:2011JChEd..88.1694S. doi:10.1021/ed200096e.
- ^ Y. Cudennec, A. Lecerf (2003). "The transformation of Cu(OH)ii into CuO, revisited" (PDF). Solid State Sciences. 5 (11–12): 1471–1474. Bibcode:2003SSSci...v.1471C. doi:x.1016/j.solidstatesciences.2003.09.009. S2CID 96363475.
- ^ H. R. Oswald, A. Reller, H. West. Schmalle, Due east. Dubler (1990). "Structure of Copper(II) Hydroxide, Cu(OH)ii". Acta Crystallogr. C46 (12): 2279–2284. doi:x.1107/S0108270190006230.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ^ Y. Cudennec; et al. (1995). "Etude cinétique de l'oxydation de l'ammoniac en présence d'ions cuivriques". Comptes Rendus de fifty'Académie des Sciences, Série IIB. 320 (half-dozen): 309–316.
- ^ Y. Cudennec; et al. (1993). "Synthesis and written report of Cu(NOii)two(NH3)4 and Cu(NO2)2(NH3)ii". European Journal of Solid Land and Inorganic Chemistry. xxx (one–2): 77–85.
- ^ Pauling, Linus (1970). General Chemistry. Dover Publications, Inc. (p 702).
- ^ a b Tsuda, T. (2001). "Copper(II) Hydroxide". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rc228. ISBN0471936235.
- ^ Bordeaux Mixture. UC IPM online. 2007.
- ^ "SePRO Corporation".
- ^ Zigan, F.; Schuster, H.D. (1972). "Verfeinerung der Struktur von Azurit, Cuiii(OH)2(CO3)2, durch Neutronenbeugung". Zeitschrift für Kristallographie, Kristallgeometrie, Kristallphysik, Kristallchemie. 135 (five–6): 416–436. Bibcode:1972ZK....135..416Z. doi:x.1524/zkri.1972.135.5-6.416. S2CID 95738208.
- ^ Kondinski, A.; Monakhov, K. (2017). "Breaking the Gordian Knot in the Structural Chemistry of Polyoxometalates: Copper(II)–Oxo/Hydroxo Clusters". Chemistry: A European Journal. 23 (33): 7841–7852. doi:10.1002/chem.201605876. PMID 28083988.
External links [edit]
- Material Prophylactic Data Sheet
Formula For Copper Ii Hydroxide,
Source: https://en.wikipedia.org/wiki/Copper%28II%29_hydroxide
Posted by: richardswalouteemper.blogspot.com



0 Response to "Formula For Copper Ii Hydroxide"
Post a Comment